Activity 8: Exploration of Chemistry
For activity 8, students have a choice to explore other topics of chemistry presented in the PhET simulations.
Tasks to be completed:
1. Choose any Teaching Idea from any of the Chemistry Simulations (http://phet.colorado.edu/en/simulations/category/chemistry ) and post your results/data and/or answers on your blog.
Chosen activity: Build a Molecule
Teaching Idea: Concept questions
1. A
2. D
3. A
4. B
5. A
6. D
7. C
Sunday, July 10, 2011
Activity #7
Activity 7: Acids and Bases
Water is everywhere! So, lets spend one more activity learning about one of the key aspects of water. Water has the ability to dissociate (break apart from HOH (or H2O) into H+ ions and OH- ions). We refer to solutions with lots of H+ ions as acids and solutions with lots of OH- ions as bases. By adding chemicals with H+ ions acidic solutions can be made. By adding chemicals with OH- ions basic solutions can be made.
Activity Tasks:
1. Review the Content Slides Acids and Bases on the D2L site.
2. Complete the Teaching Idea “Concept Questions for Chemistry using PhET” posted by Trish Loeblein on the pH Scale simulation at PHET (http://phet.colorado.edu/en/simulation/ph-scale). On your blog post the answers with your scientific explanations from the “Clicker Questions pH Scale” posted by Trish.
1. A
2. D
3. E
4. B
5. D
6. A
7. B
8. A
9. C
10. A
3. Complete the Teaching Idea “Intro to Strong and Weak Acids and Bases” posted by Chris Bires on the Acid-Base Solutions simulation (http://phet.colorado.edu/en/simulation/acid-base-solutions) and post on your blog your data and answers to the questions posed.



Water is everywhere! So, lets spend one more activity learning about one of the key aspects of water. Water has the ability to dissociate (break apart from HOH (or H2O) into H+ ions and OH- ions). We refer to solutions with lots of H+ ions as acids and solutions with lots of OH- ions as bases. By adding chemicals with H+ ions acidic solutions can be made. By adding chemicals with OH- ions basic solutions can be made.
Activity Tasks:
1. Review the Content Slides Acids and Bases on the D2L site.
2. Complete the Teaching Idea “Concept Questions for Chemistry using PhET” posted by Trish Loeblein on the pH Scale simulation at PHET (http://phet.colorado.edu/en/simulation/ph-scale). On your blog post the answers with your scientific explanations from the “Clicker Questions pH Scale” posted by Trish.
1. A
2. D
3. E
4. B
5. D
6. A
7. B
8. A
9. C
10. A
3. Complete the Teaching Idea “Intro to Strong and Weak Acids and Bases” posted by Chris Bires on the Acid-Base Solutions simulation (http://phet.colorado.edu/en/simulation/acid-base-solutions) and post on your blog your data and answers to the questions posed.



Saturday, July 9, 2011
Activity #6
Activity 6: States of Matter and Intermolecular Forces
1. Convert 0°F, 32°F, 70°F, and 212°F to Kelvin
0°F=255.37
32°F=273.15
70°F=294.26
212°F=373.15
4. Switch to the Phase Changes Tab on the States of Matter simulation. Notice how on the bottom right there is a small red dot that indicates where the system is at as far as temperature, pressure and state of matter. Play with the simulation to notice changes, notice that when you push down the pressure can go way up and explode the box. On your blog, report a temperature and pressure required to make oxygen a liquid. This is sometimes how the oxygen exists in pressurized oxygen tanks, perhaps like ones you may use to go diving.
1. Convert 0°F, 32°F, 70°F, and 212°F to Kelvin
0°F=255.37
32°F=273.15
70°F=294.26
212°F=373.15
2. Complete the Teaching Idea: States of Matter Simulation Lab by Kelly Vaughan. Complete the lab worksheet as if you were a student, and then post this on your blog. You can scan it or just take a picture of it.
3. In the States of Matter simulation, choose the Solid, Liquid, and Gas Tab at the top of the screen. Choose the water molecule and cool the water to 0 K. Describe how the water molecules are aligned and attracted to each other. Which atoms are attracted to which other atoms?
The oxygen molecules are attracted to each other. The molecules hardly move.
4. Switch to the Phase Changes Tab on the States of Matter simulation. Notice how on the bottom right there is a small red dot that indicates where the system is at as far as temperature, pressure and state of matter. Play with the simulation to notice changes, notice that when you push down the pressure can go way up and explode the box. On your blog, report a temperature and pressure required to make oxygen a liquid. This is sometimes how the oxygen exists in pressurized oxygen tanks, perhaps like ones you may use to go diving.
Temperature: 493 K
Pressure: 19-20 ATM
5. List and describe at least two Science Standards that this activity addresses
Standard A: Science as inquiry for grades 5-8 and 9-12
Standard B: physical science for grades 5-8 and 9-12
Activity #5
To complete Activity 5, complete the tasks below:
1. Run the Build an Atom simulation http://phet.colorado.edu/en/simulation/build-an-atom and build a neutral lithium atom and a neutral boron atom. Take a picture, or a screen shot, of these two atoms and place them on your blog. List the number of protons, neutrons and electrons for each. Also look up and post the density for each of the elements on your blog.
Lithium:
protons=3
neutrons=3
electrons=3
density= 0.53
2. Define density and the equation for density and post on your blog.
Density is the quantity of mass per unit volume
Density = mass/volume
3. Run the Density simulation http://phet.colorado.edu/en/simulation/density and complete one(your choice) of the prepared Teaching Ideas and post your results on your blog. The activity you choose should be one of the student intended activities.
Chosen activity: Relative Density-Sink of Float
Wood: Mass= 2.00 kg
Volume= 5.00 L
Density= 0.40 kg/L
Floats!!
Ice: Mass= 4.60 kg
Volume= 5.00 L
Density= 0.29 kg/L
Alsmot sinks, still afloat a little bit!
Brick: Mass= 10.00 kg
Volume= 5.00 L
Density= 2.00 kg/L
Sinks!!!
Aluminum: Mass= 13.50 kg
Volume= 5.00 L
Density= 2.70 kg/L
Sinks!!!
4. Complete the Mystery Blocks activity on the Density simulation. Post on your blog the data you collected (mass, volume, and density) and the identification of the material and the known density.
.
5. Identify and post on your blog the Science Standards that could be met through these activities completed in Activity 5
For the density simulation I completed standard A: Science as inquiry for grades 5-8 and 9-12
and standard B: physical science for grades 5-8 and 9-12
1. Run the Build an Atom simulation http://phet.colorado.edu/en/simulation/build-an-atom and build a neutral lithium atom and a neutral boron atom. Take a picture, or a screen shot, of these two atoms and place them on your blog. List the number of protons, neutrons and electrons for each. Also look up and post the density for each of the elements on your blog.
Lithium:
protons=3
neutrons=3
electrons=3
density= 0.53
Boron:
protons=5
neutrons=6
electrons=5
density= 2.34
Density is the quantity of mass per unit volume
Density = mass/volume
3. Run the Density simulation http://phet.colorado.edu/en/simulation/density and complete one(your choice) of the prepared Teaching Ideas and post your results on your blog. The activity you choose should be one of the student intended activities.
Chosen activity: Relative Density-Sink of Float
Wood: Mass= 2.00 kg
Volume= 5.00 L
Density= 0.40 kg/L
Floats!!
Ice: Mass= 4.60 kg
Volume= 5.00 L
Density= 0.29 kg/L
Alsmot sinks, still afloat a little bit!
Brick: Mass= 10.00 kg
Volume= 5.00 L
Density= 2.00 kg/L
Sinks!!!
Aluminum: Mass= 13.50 kg
Volume= 5.00 L
Density= 2.70 kg/L
Sinks!!!
4. Complete the Mystery Blocks activity on the Density simulation. Post on your blog the data you collected (mass, volume, and density) and the identification of the material and the known density.
Volume | Mass | Density | Object |
3.38 | 65.14 | 19.27 | Gold |
.64 | .64 | 1 | Water |
4.08 | 4.08 | 1 | Water |
3.08 | 3.10 | .99 | Water |
1.00 | 3.53 | 3.53 | Diamond |
.
5. Identify and post on your blog the Science Standards that could be met through these activities completed in Activity 5
For the density simulation I completed standard A: Science as inquiry for grades 5-8 and 9-12
and standard B: physical science for grades 5-8 and 9-12
Friday, July 8, 2011
Activity #4
Sub Standard A:
A.4.5 When studying a science-related problem, decide what changes over time are occurring or have occurred
This is something that children learn very early in school. When I was young I remember studying catterpillars in school and observing and describing the different chances the caterpillar went through during its journey in becoming a butterfly. These changes occur overtime and we learned to document these changes and relate the process to other things in nature and in science.
Sub Standard B:
B.4.1 Use encyclopedias, source books, texts, computers, teachers, parents, other adults, journals, popular press, and various other sources, to help answer science-related questions and plan investigations
Growing up in this day and age we were very lucky as to have things like the internet to help us answer questions about anything. But when we were kids in school we were taught to not use the internet but infact look things up in encyclopedias and books to find what we were looking for. I can remember getting an assignment where we had to find the answers to all of the questions in books and other texts and we were not allowed to use the internet at all. Everyone should know how to use encyclopedias and books to find information even if we do have the internet and google at the press of a button. Its just good information to know.
Sub Standard C:
C.4.4 Use simple science equipment safely and effectively, including rulers, balances, graduated cylinders, hand lenses, thermometers, and computers, to collect data relevant to questions and investigations
This is something I have used in every science class I have ever been in from grade school through college. We learned how to use these instruments when we were in elementary school and its knowledge that you will use all the way through college. In my chemistry class my senior year of high school we did experiemnts twice a week and used all of the instruments mentioned in this question plus many more. Everyone needs to know how to use these and once you learn you will apply it to many other things in your life.
Sub Standard D:
D.4.3. Understand that substances can exist in different states-solid, liquid, gas
This question was justed used in the first activity in this course. The questions we answered had to do with water and the different states of water and freezing and boiling points. Water isnt the only substance that can occur in different forms, however it may be one of the only few that exist in all three forms.
Sub Standard E:
E.4.4 Identify celestial objects (stars, sun, moon, planets) in the sky, noting changes in patterns of those objects over time
I took an astronomy class in high school and I loved it. Space and the stars and planets have always fascinated me and it is my favorite area in science to learn about. The information I learned in my astronomy class has carried with me and it helped me learn about the stars and planets. I use it most when im looking for constellations and I can now identify most of them. As kids we learned the planets and some constellations, but getting older and learning more you learn how the earth rotates and you see different constellations during different times of the year.
Sub Standard F:
F.4.3 Illustrate* the different ways that organisms grow through life stages and survive to produce new members of their type
I can remember having to do drawings of things we see in nature and their life cycles in school. For instance one experiment I can remember doing was planting a seed and drawing an illustration of the seed and planting it in the dirt. Throughout the life cycle of the seed we would document and draw what we were seeing during each cycle all the way through its flowering and giving off new seeds to make new flowers.
Sub Standard G:
G.4.2 Discover* what changes in technology have occurred in a career chosen by a parent, grandparent, or an adult friend over a long period of time
Technology changes every single day and it is hard to keep up with. All careers these days use technology and it is important to stay up to date with programs and software to help everyone be efficient. Especially in medicine technological advances are being made every day and hospitals need to be aware and alert of the changes going on and having the latest technology and machines to give people the best possible care. My aunt is a Radiologist and if ultrasound or CT machines were never updated then we would not have the amazing information and knowledge that we have now as far as radiology and other fields in medicine go.
Sub Standard H:
H.4.1 Describe* how science and technology have helped, and in some cases hindered, progress in providing better food, more rapid information, quicker and safer transportation, and more effective health care
Like I said in the previous question technology helps us every day. In food it is very important because it is finding ways to keep food safer for longer and preservation is very important. We have all these wonderful uses of technology these days that we didnt have years ago and it is providing ways for us to constantly learn about food and safety which helps everyone. See above for health care.
A.4.5 When studying a science-related problem, decide what changes over time are occurring or have occurred
This is something that children learn very early in school. When I was young I remember studying catterpillars in school and observing and describing the different chances the caterpillar went through during its journey in becoming a butterfly. These changes occur overtime and we learned to document these changes and relate the process to other things in nature and in science.
Sub Standard B:
B.4.1 Use encyclopedias, source books, texts, computers, teachers, parents, other adults, journals, popular press, and various other sources, to help answer science-related questions and plan investigations
Growing up in this day and age we were very lucky as to have things like the internet to help us answer questions about anything. But when we were kids in school we were taught to not use the internet but infact look things up in encyclopedias and books to find what we were looking for. I can remember getting an assignment where we had to find the answers to all of the questions in books and other texts and we were not allowed to use the internet at all. Everyone should know how to use encyclopedias and books to find information even if we do have the internet and google at the press of a button. Its just good information to know.
Sub Standard C:
C.4.4 Use simple science equipment safely and effectively, including rulers, balances, graduated cylinders, hand lenses, thermometers, and computers, to collect data relevant to questions and investigations
This is something I have used in every science class I have ever been in from grade school through college. We learned how to use these instruments when we were in elementary school and its knowledge that you will use all the way through college. In my chemistry class my senior year of high school we did experiemnts twice a week and used all of the instruments mentioned in this question plus many more. Everyone needs to know how to use these and once you learn you will apply it to many other things in your life.
Sub Standard D:
D.4.3. Understand that substances can exist in different states-solid, liquid, gas
This question was justed used in the first activity in this course. The questions we answered had to do with water and the different states of water and freezing and boiling points. Water isnt the only substance that can occur in different forms, however it may be one of the only few that exist in all three forms.
Sub Standard E:
E.4.4 Identify celestial objects (stars, sun, moon, planets) in the sky, noting changes in patterns of those objects over time
I took an astronomy class in high school and I loved it. Space and the stars and planets have always fascinated me and it is my favorite area in science to learn about. The information I learned in my astronomy class has carried with me and it helped me learn about the stars and planets. I use it most when im looking for constellations and I can now identify most of them. As kids we learned the planets and some constellations, but getting older and learning more you learn how the earth rotates and you see different constellations during different times of the year.
Sub Standard F:
F.4.3 Illustrate* the different ways that organisms grow through life stages and survive to produce new members of their type
I can remember having to do drawings of things we see in nature and their life cycles in school. For instance one experiment I can remember doing was planting a seed and drawing an illustration of the seed and planting it in the dirt. Throughout the life cycle of the seed we would document and draw what we were seeing during each cycle all the way through its flowering and giving off new seeds to make new flowers.
Sub Standard G:
G.4.2 Discover* what changes in technology have occurred in a career chosen by a parent, grandparent, or an adult friend over a long period of time
Technology changes every single day and it is hard to keep up with. All careers these days use technology and it is important to stay up to date with programs and software to help everyone be efficient. Especially in medicine technological advances are being made every day and hospitals need to be aware and alert of the changes going on and having the latest technology and machines to give people the best possible care. My aunt is a Radiologist and if ultrasound or CT machines were never updated then we would not have the amazing information and knowledge that we have now as far as radiology and other fields in medicine go.
Sub Standard H:
H.4.1 Describe* how science and technology have helped, and in some cases hindered, progress in providing better food, more rapid information, quicker and safer transportation, and more effective health care
Like I said in the previous question technology helps us every day. In food it is very important because it is finding ways to keep food safer for longer and preservation is very important. We have all these wonderful uses of technology these days that we didnt have years ago and it is providing ways for us to constantly learn about food and safety which helps everyone. See above for health care.
Activity #3
Questions/Activities:
1. Post a picture of three 3-dimensional Ball and Stick molecular models(choose your three favorite molecules) that you have created with common items around your home. Also post a molecular structure image(image from the web, of either a Kekule Structure or a Ball and Stick Model) and the IUPAC name of the molecule.
ETHANOL
METHANE
2. Post an image from the web, the chemical systematic (IUPAC) name, common name, and the molecule formula for 20 chemicals that you use or eat. Explore the ingredients of things like cosmetics and foods.
Graphite=pencils

Lithium is in batteries
 copper-pennies and coins
copper-pennies and coins oxygen
oxygen aluminum-cans
aluminum-cans  iron-foods like meat and vegetables
iron-foods like meat and vegetables  butanoic acid
butanoic acid  ascorbic acid-vitamind C
ascorbic acid-vitamind C calcium carbonate
calcium carbonate
 fructose-foods and drinks
fructose-foods and drinks 
polyethylene
 cirtric acid-juices like orange juice
cirtric acid-juices like orange juice  oxidane
oxidane 3. Look over your molecules and the bonding characteristics, how many bonds does each of the following elements typically have? Carbon? Hydrogen? Oxygen?
Carbon: 4
Hydrogen: 1
Oxygen: 2
4. What does IUPAC stand for?
International Union of Pure and Applied Chemistry
5. As you explore ingredients, notice how everything around us is made up of chemicals consisting of atoms bound together into molecules. But what about companies that claim their products are chemical free! How can this be? Here is an example:
http://www.naturalhealthcareproducts.com/Cleaning-Products.php
Do a little web searching and propose what chemicals are actually in this product.
There are products like Lysol and Clorox that use harsh chemicals like bleach and amonia to kill germs and bacteria, and companies like those are starting to come out with "chemical free" or "safe" products. using less-harsh chemicals in your products do make them safer to use around children or food, but there can never be a "chemical free" product. All cleaning products will have some sort of chemical in them to kill the germs, but it just might not be as harsh as bleach.
Wednesday, July 6, 2011
Activity #2
Activity 2: Atom and Atomic Structure
For this activity review the Content Slides (Atoms and Atomic Structure) and complete the activities/questions below.
Make a model of your three favorite elements on the Periodic Chart. The models must be 3-dimensional and be made out of common objects around your home. Place photos of your three models with descriptions on your blog. Your models must include the appropriate number and positioning of protons, neutrons and electrons.
For this activity review the Content Slides (Atoms and Atomic Structure) and complete the activities/questions below.
Make a model of your three favorite elements on the Periodic Chart. The models must be 3-dimensional and be made out of common objects around your home. Place photos of your three models with descriptions on your blog. Your models must include the appropriate number and positioning of protons, neutrons and electrons.
Protons | Neutrons | Electrons | |
| Oxygen | 8 | 8 | 8 |
| Carbon | 6 | 6 | 6 |
| Helium | 2 | 2 | 2 |
Questions:
1. What is the atomic number for each of your models?
Oxygen: 8
1. What is the atomic number for each of your models?
Oxygen: 8
Helium: 2
Carbon: 6
2. What is the atomic mass number for each of your models?
Oxygen: 15.9994
Oxygen: 15.9994
Helium: 4.0026
Carbon: 12.0107
3. In your models, which two subatomic particles are equal in number?
none of which are equal in number. Each one is equal in itself with protons, electrons, and neutrons.
none of which are equal in number. Each one is equal in itself with protons, electrons, and neutrons.
4. How would you make an isotope for one of your models? What would change with the model?If you changed the number of neutrons in the oxygen molecule it would make the nucleus unstable
5. Considering the overall volume of your element models, what makes up most of the volume of an atom?
The Nucleus
The Nucleus
6. For one of your models, show with another image what happens when energy excites an electron.
7. Once the electron is excited, what do we typically observe when the electron returns to the ground-state?
Goes back to its original state.
Goes back to its original state.
8. Why are some elements different colors when they are excited?
The energy they emit can come in the form of light. Its like the experiment that we did in high school where we would light different elements on fire to see what color they burned. The light we saw was because the electrons were excited.
The energy they emit can come in the form of light. Its like the experiment that we did in high school where we would light different elements on fire to see what color they burned. The light we saw was because the electrons were excited.
9. With the Fourth of July coming up quickly, explain how the colors of fireworks arise.
| Color | Compound |
| Red | strontium salts, lithium salts lithium carbonate, Li2CO3 = red strontium carbonate, SrCO3 = bright red |
| Orange | calcium salts calcium chloride, CaCl2 calcium sulfate, CaSO4·xH2O, where x = 0,2,3,5 |
| Gold | incandescence of iron (with carbon), charcoal, or lampblack |
| Yellow | sodium compounds sodium nitrate, NaNO3 cryolite, Na3AlF6 |
| Electric White | white-hot metal, such as magnesium or aluminum barium oxide, BaO |
| Green | barium compounds + chlorine producer barium chloride, BaCl+ = bright green |
| Blue | copper compounds + chlorine producer copper acetoarsenite (Paris Green), Cu3As2O3Cu(C2H3O2)2 = blue copper (I) chloride, CuCl = turquoise blue |
| Purple | mixture of strontium (red) and copper (blue) compounds |
| Silver | burning aluminum, titanium, or magnesium powder or flakes |
| Color | Compound |
| Red | strontium salts, lithium salts lithium carbonate, Li2CO3 = red strontium carbonate, SrCO3 = bright red |
| Orange | calcium salts calcium chloride, CaCl2 calcium sulfate, CaSO4·xH2O, where x = 0,2,3,5 |
| Gold | incandescence of iron (with carbon), charcoal, or lampblack |
| Yellow | sodium compounds sodium nitrate, NaNO3 cryolite, Na3AlF6 |
| Electric White | white-hot metal, such as magnesium or aluminum barium oxide, BaO |
| Green | barium compounds + chlorine producer barium chloride, BaCl+ = bright green |
| Blue | copper compounds + chlorine producer copper acetoarsenite (Paris Green), Cu3As2O3Cu(C2H3O2)2 = blue copper (I) chloride, CuCl = turquoise blue |
| Purple | mixture of strontium (red) and copper (blue) compounds |
| Silver | burning aluminum, titanium, or magnesium powder or flakes |
fireworks have all different kinds of elements in them which when exited give off different colors like I explained above. Red fireworks have strontium and lithium, orange have calcium, yellow has sodium, green has barium and chlorine, etc..
10. Explain the overall organizational structure of the periodic table.
The verticle rows are called familes or groups, and they contain elements that are similar in chemical properties. Then the rows going horizontally are called periods which are organized by atomic number and mass
The verticle rows are called familes or groups, and they contain elements that are similar in chemical properties. Then the rows going horizontally are called periods which are organized by atomic number and mass
11. List two example elements for each of these groups or classes: Alkali Metals, Alkaline Earth, Halogens, Noble Gases, Transition Metals, Non-Metals, and Metalloids.
-Alkali Metals: lithium and sodium
-Alkaline Earth: magnesium and calcium
-Halogens: chlorine and bromine
-Noble Gases: helium and neon
-Transition Metals: Sodium and copper
-Non metals: sulfur and bromine
-Metalloids: silicon and arsenic
Tuesday, July 5, 2011
Activity #1
Here are three experimental questions to answer:
1.) Does hot water or cold water freeze faster?
answer: cold
2.) Does hot water or cold water boil faster?
answer: They boil at the same rate
5. List your controlled variables for your experiment
My controlled variables include always using the same water (tap water), using the same pots and pans for the boiling part of the experiment, same burnders used, same cups used to freeze the different kinds of water, etc.
6. Formulate a theory that answers the questions posed.

9. Describe the scientific method/process and how each step correlates to your own experiments.
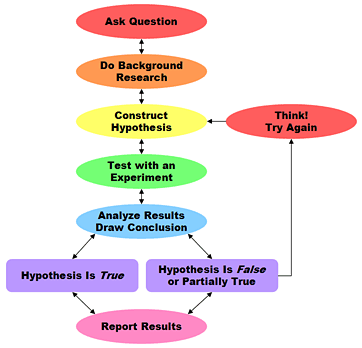
1.) Does hot water or cold water freeze faster?
answer: cold
2.) Does hot water or cold water boil faster?
answer: They boil at the same rate
3.) Does salt water freeze faster or slower than regular water?
answer: slower
Questions/Items to then include in you blog posting for this activity:
1. Pictures of your experimental materials and setup.
2. Your hypothesis to the questions posed.I predict that the cold water will freeze faster because the water is already cold. The hot water has a bigger temperature differnce to reach before freezing, but the cold water is already cold. Going along the same lines, I also predict that the hot water will boil faster because its temperature is already warm. And finally I predict that the salt water will freeze slower than regular water because when you think about it you never see salt water in nature frozen.
3. Data in the form of a graph or table
Freezing time | Boiling time | |
| 1 cup at hot water | 1 hr. 26 min. | 3 min. 36 sec. |
| 1 cup of cold water | 1 hr. 3 min. | 3 min. 36 sec. |
| 1 cup of cold water plus an added teaspoon of salt | 1 hr. 35 min. | N/A |
4. Show data of experiment repeated
Freezing time | Boiling time | |
| 1 cup at hot water | 1 hr. 24 min. | 3 min. 25 sec. |
| 1 cup of cold water | 1 hr. 10 min. | 3 min. 28 sec. |
| 1 cup of cold water plus an added teaspoon of salt | 1 hr. 38 min. | N/A |
5. List your controlled variables for your experiment
My controlled variables include always using the same water (tap water), using the same pots and pans for the boiling part of the experiment, same burnders used, same cups used to freeze the different kinds of water, etc.
Water=boiling when I can see bubbles on the surface
Water=frozen when the first layer of water in the glass is frozen
6. Formulate a theory that answers the questions posed.
It does not matter what temp the water starts out while boiling because hot water and cold water freeze at the same rate
The temp the water starts out at when freezing water does not matter because even though the cold water will freeze faster, both the hot water and the cold water will eventually freeze throughout.
Large amounts of salt water, like the ocean, will never freeze due to the large mass of it. At first the salt water will freeze slower than water without salt, but it will turn into a more slushly like consistancy while the non salt water will be hard like an ice cube.
7. Image of the atoms that make up water molecules.
8. Video or animation that shows how water molecules are arranged in the three states of matter for water.

9. Describe the scientific method/process and how each step correlates to your own experiments.

Subscribe to:
Posts (Atom)